IMVIC TEST
(Indole Test, Methyl Red Test, Voges Proskauer Test and Citrate Test)
The IMViC Tests
The IMViC tests are used to differentiate the enterics (Family Enterobacteriaceae). These are the Indole test (tryptone broth), the Methyl Red and Voges-Proskauer tests (MRVP broth) and the Citrate test (Citrate agar slants). For these IMViC tests use the enterics E.coliand Enterobacter.
The significance of these tests is that when testing drinking water for the presence of the sewage indicatorE. coli, one must be able to rule out Enterobacter aerogenes.E. aerogenesis not always associated with sewage, and its presence in water would not necessarily indicate sewage contamination.
Indole Test (Tryptone broth)
Procedure: Inoculate a loopful of bacteria into a tryptone broth. Incubate 48 hours.
Description: Tryptophan hydrolysis -Some bacteria split tryptophan into indole and pyruvic acid using the hydrolase called tryptophanase. Indole can be detected with Kovac's reagent (Indole reagent). This test is very important in differentiating E. coli(indole positive) from some closely related enteric bacteria. It also differentiatesProteus mirabilis(indole negative) from all otherProteusspecies (indole positive). Tryptone broth is used for this test as it contains a large amount of tryptophan.
Interpretation: After incubation: The broth must be turbid. A clear broth indicates that your organism did not grow and cannot be tested. Add a few drops of Indole reagent to the broth culture (tryptone broth). DO NOT SHAKE THE TUBE. A positive result has a red layer at the top. A negative result has a yellow or brown layer.
Methyl Red Test (MRVP broth)
Procedure: Inoculate a loopful of bacteria into MRVP broth. Incubate 3 to 5 days.
Description: Mixed acid fermentation -Many gram-negative intestinal bacteria can be differentiated based on the products produced when they ferment the glucose in MR-VP medium. Escherichia, Salmonella, andProteusferment glucose to produce lactic, acetic, succinic, and formic acids and CO2, H2, and ethanol. The large amounts of acids produced lowers the pH of the medium -Methyl red (a pH indicator) will turn red when added to the medium if the organism was a mixed acid fermenter. Many of these organisms also produce gas.
Interpretation: After incubation: The broth must be turbid. A clear broth indicates that your organism did not grow and cannot be tested. Remove 1 ml of broth and place into a sterile tube before performing the methyl red test ifyou are going to use the same broth for the VP test. Add 3-4 drops of methyl red to the original broth. DO NOT SHAKE THE TUBE. A positive result has a distinct red layer at the top of the broth. A negative result has a yellow layer.
Voges-Proskauer Test
Procedure: Inoculate a loopful of bacteria into MRVP broth. Incubate 3 to 5 days.
Description: Organisms that are negative in the methyl red test may be producing 2, 3 butanediol and ethanol instead of acids. These non-acid products do not lower the pH as much as acids do.Enterobacter, Serratiaand some species of Bacillus produce these substances. There is no satisfactory test for determining production of 2, 3 butanediol. A precursor of 2,3 butanediol called acetoin can be detected with Barritt's reagent.
Interpretation: After incubation: Read the VP test when you have good turbidity. A clear broth indicates that your organism did not grow and cannot be tested. Barritt's reagent A (VP A) contains naphthol and Barritt's B (VP B) contains KOH. Test 1 ml of your culture from the MRVP broth. If you have already conducted the methyl red test, you should have already placed 1 ml of untested broth in a sterile tube. If you haven’t done this, do so now. Add the entire contents of the VP A reagent (15 drops) and 5 drops of the VP B reagent to the 1 ml of your broth culture. SHAKE WELL. This reaction will take a few minutes before you will see a color change. SHAKE the tube every few minutes for best results. With a positive reaction the medium will change to pink or red indicating that acetoin is present. With a negative reaction the broth will not change color or will be copper colored. Wait at least 15 minutes for color to develop before calling the test negative.
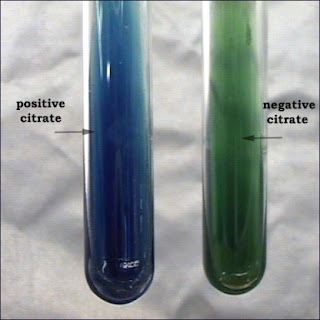
Citrate Test (Simmon's Citrate Slant)
Procedure: Streak a loopful of bacteria onto a citrate agar slant, do notstab the butt. Incubate 24 to 48 hours, longer forBacillus species. Incubate with a loose cap.
Description: Simmon's citrate agar tests for the ability of an organism to use citrate as its sole source of carbon. This media contains a pH indicator called bromthymol blue. The agar media changes from green to blue at an alkaline pH.
Interpretation: After incubation: A positive reaction is indicated by a slant with a Prussian blue color. A negative slant will have no growth of bacteria and will remain green.
IMViC TEST
INSTAGRAM
FACEBOOK








Hiç yorum yok:
Yorum Gönder