peptidoglycan
Ocak 31, 2019
Gram Negative Bacteria
Gram Negative Bacteria
Gram negative bacteria are bacteria that do not retain the crystal violet dye in the Gram stain protocol. Gram negative bacteria will thus appear red or pink following a Gram stain procedure due to the effects of the counterstain (for example safranin).
The Gram Stain
In microbiology, the visualization of bacteria at the microscopic level is facilitated by the use of stains, which react with components present in some cells but not others. This technique is used to classify bacteria as either Gram-positive or Gram-negative depending on their colour following a specific staining procedure originally developed by Hans Christian Gram. Gram-positive bacteria appear dark blue or violet due to the crystal violet stain following the Gram stain procedure; Gram-negative bacteria, which cannot retain the crystal violet stain, appear red or pink due to the counterstain (usually safranin).
The reason bacteria are either Gram-positive or Gram-negative is due to the structure of their cell envelope. (The cell envelope is defined as the cell membrane and cell wall plus an outer membrane, if one is present.) Gram-positive bacteria, for example, retain the crystal violet due to the amount of peptidoglycan in the cell wall. It can be said therefore that the Gram-stain procedure separates bacteria into two broad categories based on structural differences in the cell envelope.
Cell Envelope of Gram Negative Bacteria
The Gram negative cell envelope contains an additional outer membrane composed by phospholipids and lipopolysaccharides which face the external environment. The highly charged nature of lipopolysaccharides confer an overall negative charge to the Gram negative cell wall. The chemical structure of the outer membrane lipopolysaccharides is often unique to specific bacterial strains (i.e. sub-species) and is responsible for many of the antigenic properties of these strains. Many species of Gram-negative bacteria are pathogenic. This pathogenicity is often associated with the lipopolysaccharide (LPS) layer of the Gram-negative cell envelope.
Characteristics of Gram Negative Bacteria
Gram-negative bacteria have a characteristic cell envelope structure very different from Gram-positive bacteria. Gram-negative bacteria have a cytoplasmic membrane, a thin peptidoglycan layer, and an outer membrane containing lipopolysaccharide. There is a space between the cytoplasmic membrane and the outer membrane called the periplasmic space or periplasm. The periplasmic space contains the loose network of peptidoglycan chains referred to as the peptidoglycan layer.
- Acinetobacter
- Actinobacillus
- Bordetella
- Brucella
- Campylobacter
- Cyanobacteria
- Enterobacter
- Erwinia
- Escherichia coli
- Franciscella
- Helicobacter
- Hemophilus
- Klebsiella
- Legionella
- Moraxella
- Neisseria
- Pasteurella
- Proteus
- Pseudomonas
- Salmonella
- Serratia
- Shigella
- Treponema
- Vibrio
- Yersinia
INSTAGRAM
28 Ocak 2019 Pazartesi
mikrobiyoloji
Ocak 28, 2019
Bacillus anthracis (Anthrax)
Bacillus anthracis (Anthrax)
26 Ocak 2019 Cumartesi
Riketsiyalar
Ocak 26, 2019
Riketsiyalar (Riketsiya) (Rickettsia)
Riketsiyalar (Riketsiya)
Riketsiyalar küçük, Gram-negatif, kok veya çubuk şeklinde olup genişlikleri 0,3-0,7
mikrometre, uzunlukları da 1-2 mikrometredir. Bir istisna dışında zorunlu hücre içi paraziti
olup konukçu hücre yokluğunda kültür edilememiştir. Bu nedenle bazı kaynaklarda virüs ve
bakteri arası geçiş formu olarak bildirilmiştir.
Riketsiyaların Genel Özellikleri
Riketsiyalar, insanlarda hastalıklara sebep olur.Tüm dünyada yaygın bulunmalarına
karşılık farklı coğrafi bölgelerde değişik cinsleri bulunur. İnsanlara sindirim sistemlerinde
parazit olarak yaşadıkları bit, pire, kene ve akar gibi eklembacaklılar tarafından bulaştırılır.
Riketsiyaların sebep olduğu hastalıklara riketsiyoz adı verilir. Dünyanın değişik
coğrafi bölgelerinde farklı riketsiyozlar görülür. Ülkemizin de yer aldığı Akdeniz
Bölgesi’nde, Avrupa ve Afrika’da en sık Marsilya ateşi hastalığı görülür. Riketsiyozlar,
ateşle seyreden klinik tablolara yol açar. En iyi bilinen türü Rickettsia ricketsii’dir.
Riketsiyaların Virüs ve Bakterilerden Farkı
Riketsiyaların yer aldığı gruptaki mikroorganizmalar, küçük olmaları ve gram boyama
ile iyi boyanmamaları, ökaryotik hücrelerin sitoplazmalarında yaşamlarını sürdürebilmeleri
nedeniyle önceleri virüs sanılmışlardır ancak yapısal olarak gram negatif çubuklara
benzerlikleri, DNA, RNA, enzim, ribozom içermeleri ve antibiyotiklerle inhibe edilmeleri
nedeniyle bakteriler sınıfında yer almıştır. Bu özellikleri ile virüslerden ayrılmaktadır.
Riketsiyalar bakteriler gibi normal besiyerlerinde üretilemez. Zorunlu hücre içi
paraziti oldukları için embriyolu yumurta sarısında, hücre kültürlerinde veya deney
hayvanlarında üretilebilir. Bu özellikleri ile bakterilerden ayrılmaktadır.
INSTAGRAM
25 Ocak 2019 Cuma
ulcers
Ocak 25, 2019
Helicobacter pylori
Helicobacter pylori
Morphology and Habitat:
Helicobacter pylori are spiral-shaped (or sometimes straight), Gram-negative bacilli approximately 0.5 x 3.0 micrometers in size. They are motile by means of 4-6 sheathed flagella that are attached to one pole (lophotrichous). H.pylori lives in the mucosal lining of duodenum and stomach.
Helicobacter pylori are spiral-shaped (or sometimes straight), Gram-negative bacilli approximately 0.5 x 3.0 micrometers in size. They are motile by means of 4-6 sheathed flagella that are attached to one pole (lophotrichous). H.pylori lives in the mucosal lining of duodenum and stomach.
Nomenclature:
Originally, the organism was named Campylobacter pyloridis because it was structurally similar to other Campylobacter species. C.pyloridis was later renamed Campylobacter pylori and finally named Helicobacter pylori in 1989. Australian physiologists Robin Warren and Barry Marshall were awarded the 2005 Nobel Prize in medicine for their research in the early 1980's showing that peptic ulcers were primarily caused by bacteria not stress.
Cultural Characteristics:
It is microaerophilic and grows well in fresh, moist medium with humidity at 37oC. It is cultivated on chocolate or blood agar and incubated for up to 5 days. It is catalase, oxidase, urease and phosphatase positive.
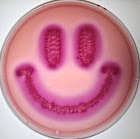
Helicobacter pylori on Blood Agar
Pathogenesis:
Source of infection: Persons suffering from H.pylori infection shed the bacilli in their feces that might contaminate food and drinking water.
Source of infection: Persons suffering from H.pylori infection shed the bacilli in their feces that might contaminate food and drinking water.
Mode of infection:
H.pylori is believed to be transmitted orally food or water. It could be transmitted from the stomach to the mouth through gastro-esophagal reflux. The bacterium could then be transmitted through oral contact. About 10% of children get infected between the ages of 2 and 8. The stomach is protected from its own gastrc juice by a thick layer of mucus that covers the stomach lining. H.pylori takes advantage of this protection by living n the mucus lining. The antrum of the stomach is a region of moderate acidity where H.pylori usually prefers to colonize first. The bacterium uses its flagella and sipral shape to drill through the mucus layer in the stomach. It is known to produce some adhesions that help it adhere to epithelial cells of mucosa. It escapes the deleterious effect of HCl in the gastric juice by producing abundant of urease. Urease converts urea, which is in abundant supply in the stomach (from saliva and gastric juices), into bicarbonate and ammonia, which serves to neutralize the acidity.
H.pylori is believed to be transmitted orally food or water. It could be transmitted from the stomach to the mouth through gastro-esophagal reflux. The bacterium could then be transmitted through oral contact. About 10% of children get infected between the ages of 2 and 8. The stomach is protected from its own gastrc juice by a thick layer of mucus that covers the stomach lining. H.pylori takes advantage of this protection by living n the mucus lining. The antrum of the stomach is a region of moderate acidity where H.pylori usually prefers to colonize first. The bacterium uses its flagella and sipral shape to drill through the mucus layer in the stomach. It is known to produce some adhesions that help it adhere to epithelial cells of mucosa. It escapes the deleterious effect of HCl in the gastric juice by producing abundant of urease. Urease converts urea, which is in abundant supply in the stomach (from saliva and gastric juices), into bicarbonate and ammonia, which serves to neutralize the acidity.
CO(NH2) 2 + H+ + 2H2O ---urease---> HCO3- + 2(NH4+)
Ammonia production from urease activity is toxic to mammalian cells. Epithelial cells undergo vacuolation because of urease acitvity. Other products of H.pylori, including protease, catalase, and phospholipases A2 and C cause weakening of the mucous layer of the GI tract and damage to surface epithelial cells. Lipopolysachaaride (LPS) may interfere with protective function of mucus layer and make epithelial cells at the surface vulnerable to acid. H.pylori is the only bacterium that stimulates pepsinogen secretion.
Body's natural defenses cannot reach the bacterium in the mucus lining of the stomach. Neutrophils and killer T cells cannot reach the infection, as they cannot easily get through stomach lining. Cytokines are produced that attract and activate inflammatory cells. These cells spill their destructive compounds (superoxide radicals) on stomach lining cells. It is the inflammaton of the stomach lining in response to H. pylori that causes peptic ulcer. Within a few days, gastritis and perhaps eventually a pepitc ulcer results. Gastritis is an infiltraton of the tissue with lymphocytes and plasma cells. Duodenal ulcers are associated with chronic superficial gastritis in the gastric antrum. Most gastric adenocarcinomas and lymphomas occur in persons with current or past infection with H. pylori.
Laboratory Diagnoss:
There are invasive and non- invasive methods to diagnose H.pylori infections.
Invasive methods include endoscopy of a patient, during which biopsies are taken from multiple sites in the oesophagus, stomach, and duodenum. The biopsy specimen must be transported to laboratory immediately in sterile saline. The sample may be refrigerated in case of delay in transportation. When there is a delay of five hours of more, Stuart's transport medium can be used.
The specimens are subjected to microscopic examination, culture and rapid urease test. The smears prepared from biopsy specimens are stained by Gram stain or Giemsa stain. The specimen is ground and inoculated on chocolate agar, blood agar or selective media such as Skirrow medium and incubated in microaerophilic conditions at 37oC. Rapid urease test checks for the presence of the enzyme urease in the biopsy tissue sample. The test is positive in as little as 10 minutes.
Non- invasive testing includes culture from the stool specimen and urea breath test. In Urea breath test, the patient drinks a solution with C-13 or C-14 labeled urea with a meal. In an infected person, labelled CO2 is detected in the breath, which is measured with a beta counter. Other methods of testing include testing for antibodies in serum or saliva of a patient. ELISA can be used to measure IgG in serum.
Click For
Treatment:
The best treatment is a triple therapy routine including bismuth subcitrate, metronidazole, and either tetracycline or amoxycillin or clarithomycin.
INSTAGRAM
24 Ocak 2019 Perşembe
üroloji
Ocak 24, 2019
İDRAR SEDİMENTİNDEN PREPARAT HAZIRLAMA (İdrar Sedimentinin Hazırlanması)
İDRAR SEDİMENTİNDEN PREPARAT HAZIRLAMA
23 Ocak 2019 Çarşamba
Protozoan
Ocak 23, 2019
Plasmodium vivax
Plasmodium vivax
P.vivax is selective in that it invades only young immature erythrocytes. Infections of P. vivax have the following characteristics:
• Infected red blood cells are usually enlarged and contain numerous pink granules or schuffner’s dots.
• The trophozoite is ring-shaped but amoeboid in appearance.
• More mature trophozoites and erythrocytic schizonts containing up to 24 merozoites are present.
• The gametocytes are round
Epidemiology
P. Vivax is the most prevalent of the human plasmodia with the widest geographic distribution, including the tropics, subtropics, and temperate regions. However, it is the second most prevalent in Ethiopia following P. falciparum.
Clinical Features
After an incubation period (usually 10 to 17 days), the patient experiences vague flu-like symptoms, such as headache, muscle pains, photophobia, anorexia, nausea and vomiting. As the infection progresses, increased numbers of rupturing erythrocytes liberate merozoites as well as toxic cellular debris and hemoglobin in to circulation. In combination, these substances produce the typical pattern chills, fever and malarial rigors. These paroxysms usually reappear periodically (generally every 48 hours) as the cycle of infection, replication, and cell lyses progresses. The paroxysms may remain relatively mild or may progress to severe attacks, with hours of sweating, chills, shaking persistently, high temperatures (1030F to 1060F) and exhaustion. Since P.vivax infects only the reticulocytes, the parasitemia is usually limited to around 2 to 5% of the available RBCs.
21 Ocak 2019 Pazartesi
sedimantasyon hızı
Ocak 21, 2019
SEDİMANTASYON (ESR) Nedir?
SEDİMANTASYON (ESR)
SEDİMANTASYON HIZI
SEDİMANTASYON HIZI
Sedim;ESR; Sed Rate;
Eritrosit Sedimantasyon Hızı:
Kan hücreleri olan eritrositlerin bir tüp içinde çökme hızlarıdır. Çok eski bir testtir ve kabaca kanda iltihabi reaksiyon varlığını gösterir. Kan alındıktan sonra bir tüp içine konur ve baş aşağı tutularak yarım saatte ve bir saatteki çökme hızları mm cinsinden yazılır. Sedimantasyonun pozitif olması spesifik bir hastalığı göstermez, birçok hastalıkta yükselebilir. laboratuvarda sedimantasyon testi Westergren metodu denen yöntemle bakılır.
Kan hücreleri olan eritrositlerin bir tüp içinde çökme hızlarıdır. Çok eski bir testtir ve kabaca kanda iltihabi reaksiyon varlığını gösterir. Kan alındıktan sonra bir tüp içine konur ve baş aşağı tutularak yarım saatte ve bir saatteki çökme hızları mm cinsinden yazılır. Sedimantasyonun pozitif olması spesifik bir hastalığı göstermez, birçok hastalıkta yükselebilir. laboratuvarda sedimantasyon testi Westergren metodu denen yöntemle bakılır.
Sedimantasyon Hızı Ne İçin Bakılır?
Kabaca vücuttaki enfeksiyonun varlığını gösteren bir testtir. Ateşli hastalıklar ve romatizmal hastalıkların araştırılması sırasında istenir. Romatizmal hastalıkların, otoimmün hastalıkların ve kronik enfeksiyonların alevlenme dönemlerinde ve bazı kanser hastalıklarında sedimantasyon artar. Hastalıkların tedaviye verdikleri cevabın izlenmesinde de sedimantasyona bakılır. Tedaviden fayda görüyor ise sedimantasyon düşmeye başlar.
Sedimantasyon Aşağıdaki Hastalıkların Takibi Sırasında Kullanılır:
- Otoimmün hastalıklar,
- Artrit ve romatizmal hastalıklar,
- Kronik enfeksiyon hastalıkları,
- Tüberküloz,
- Brusella.
Sedimantasyonun Normal Değeri Nedir?
- 50 yaş altı erkekler için saatte 15 mm altı,
- 50 yaş üstü erkekler için saatte 20 mm altı,
- 50 yaş altı kadınlar için saatte 20 mm altı,
- 50 yaş üstü kadınlar için saatte 30 mm altı normal kabul edilir.
- Çocuklar için
- Yeni doğan 0 ila 2 mm / saatte
- Süt çocukluğu – püberte döneminde 3 – 13 mm/ saatte normal sayılır.
Yüksek Sedimantasyon Ne Anlama Gelir?
Her ne kadar bazı hastalıklarda sedimantasyon yüksekliği teşhise yardım etse de tek başına sedimantasyon yüksekliği bir hastalığa işaret etmez. Sedimantasyon dışında diğer testlerin de yapılması gereklidir.
Sedimantasyon Değerini Yükselten Hastalıklar:
- Anemi,
- Lenfoma, multipl myeloma gibi bazı kanser hastalıkları,
- Böbrek hastalıkları,
- Gebelik,
- Tiroit hastalıklar.
İmmün sistemin kendi vücuduna zarar verdiği hastalıklarda sedimantasyon çok artar bu hastalıklara otoimmün hastalıklar diyoruz:
- Sistemik lupus eritematozus,
- Romatoid artrit.
Çok Yüksek Sedimantasyon En Sık Şu Hastalıklarda Görülür:
- Alerjik vaskülit,
- Dev hücreli artrit,
- Hiperfibrinojenemi,
- Makroglobülinemi,
- Nekrotizan vaskülit,
- Polimyalgia romatica.
Enfeksiyon Hastalıkları Sedimantasyonu Arttıran Diğer Bir Sebeptir:
- Sistemik enfeksiyon hastalıkları,
- Kemik enfeksiyonları, osteomyelit,
- Kalp kapağı enfeksiyonları, endokarditler,
- Romatizmal ateş,
- Ciddi cilt enfeksiyonları, erizipel,
- Tüberküloz,
- Brusella.
Sedimantasyonu Düşüren Sebepler:
- Konjestif kalp yetmezliği,
- Hiperviskozite hastalığı,
- Hipofibrinojenemi,
- Düşük plazma proteinleri (böbrek hastalıklarında görülür),
- Polisitemi,
- Orak hücreli anemi de sedimantasyon hızı düşer.